Dispelling the Myths: Common Misunderstandings About Entropy

Entropy, a concept in thermodynamics, has often been shrouded in mystery and misunderstood. From its connection to the "heat death of the universe" to its implications for information loss, various misconceptions have persisted. This article aims to dispel these myths and provide a comprehensive understanding of entropy, its true nature, and its impact on our world.
5 out of 5
| Language | : | English |
| File size | : | 46727 KB |
| Text-to-Speech | : | Enabled |
| Enhanced typesetting | : | Enabled |
| Print length | : | 328 pages |
| Lending | : | Enabled |
| Screen Reader | : | Supported |
| Paperback | : | 68 pages |
| Item Weight | : | 4.8 ounces |
| Dimensions | : | 7 x 0.16 x 10 inches |
Myth 1: Entropy is a measure of disFree Download
While entropy is often associated with disFree Download, this is not a complete or accurate definition. Entropy is more precisely a measure of the number of possible arrangements or microstates of a system. A system with a high entropy has a large number of possible microstates, while a system with low entropy has fewer possible microstates.
Consider a deck of cards. A new deck has low entropy because all the cards are Free Downloaded. As the deck is shuffled, the number of possible arrangements increases dramatically, leading to a higher entropy.
Myth 2: Entropy always increases
The second law of thermodynamics states that the entropy of an isolated system always increases over time. However, this is not always true for open or closed systems.
In an isolated system, there is no exchange of energy or matter with the surroundings. Consequently, entropy tends to increase as the system evolves towards equilibrium. In other words, the system becomes more disFree Downloaded over time.
However, in open or closed systems, entropy can decrease. For example, in a refrigerator, heat is removed from the inside, lowering the entropy of the food. In a living organism, energy is used to maintain Free Download and reduce entropy.
Myth 3: Entropy will eventually lead to the heat death of the universe
The heat death of the universe is a hypothetical state where the universe reaches maximum entropy, and all processes cease. While entropy does increase over time, this does not mean that the universe will necessarily reach this state.
The universe is a vast and complex system, and its evolution is not fully understood. It is possible that the universe will continue to expand and evolve, avoiding the heat death. Alternatively, it may eventually reach a state of maximum entropy, but this is not a certainty.

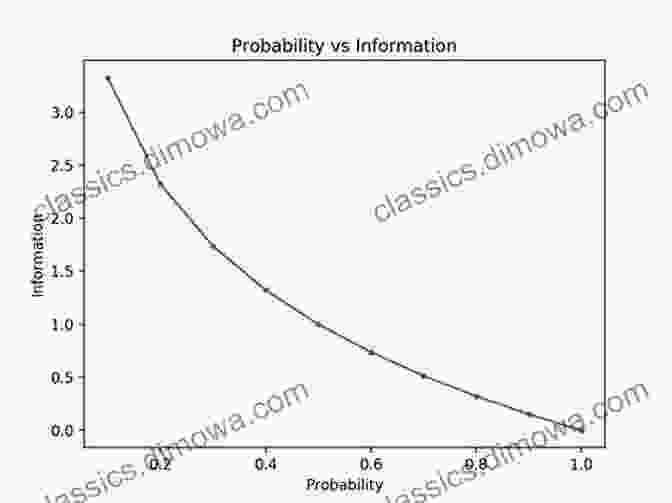
Myth 4: Entropy implies information loss
Entropy is often linked to information loss, but this is a misconception. Entropy does not necessarily indicate a decrease in information, but rather a change in its distribution.
Information can be encoded in various ways, and entropy measures the number of possible ways to encode that information. As entropy increases, the information becomes more spread out and difficult to access, but it is not necessarily lost.
Entropy is a complex and often misunderstood concept. By dispelling common misconceptions, we gain a clearer understanding of its true nature and implications. Entropy is not simply a measure of disFree Download or a sign that the universe is winding down. Instead, it is a fundamental property of systems, reflecting the number of possible arrangements of their components.
While entropy does play a role in the evolution of systems, its implications are not always straightforward. Entropy can decrease in open or closed systems, and it does not necessarily lead to the heat death of the universe or the loss of information. By embracing a more accurate understanding of entropy, we can better appreciate its significance in the world around us.
5 out of 5
| Language | : | English |
| File size | : | 46727 KB |
| Text-to-Speech | : | Enabled |
| Enhanced typesetting | : | Enabled |
| Print length | : | 328 pages |
| Lending | : | Enabled |
| Screen Reader | : | Supported |
| Paperback | : | 68 pages |
| Item Weight | : | 4.8 ounces |
| Dimensions | : | 7 x 0.16 x 10 inches |
Do you want to contribute by writing guest posts on this blog?
Please contact us and send us a resume of previous articles that you have written.
 Book
Book Novel
Novel Page
Page Chapter
Chapter Text
Text Story
Story Genre
Genre Reader
Reader Library
Library Paperback
Paperback E-book
E-book Magazine
Magazine Newspaper
Newspaper Paragraph
Paragraph Sentence
Sentence Bookmark
Bookmark Shelf
Shelf Glossary
Glossary Bibliography
Bibliography Foreword
Foreword Preface
Preface Synopsis
Synopsis Annotation
Annotation Footnote
Footnote Manuscript
Manuscript Scroll
Scroll Codex
Codex Tome
Tome Bestseller
Bestseller Classics
Classics Library card
Library card Narrative
Narrative Biography
Biography Autobiography
Autobiography Memoir
Memoir Reference
Reference Encyclopedia
Encyclopedia Navi Sorab
Navi Sorab Arx Reads
Arx Reads Joseph Crosby Lincoln
Joseph Crosby Lincoln Rocco Forino
Rocco Forino Chris Carminucci
Chris Carminucci Barbara Lehman
Barbara Lehman Paul Raeburn
Paul Raeburn Lois Ruby
Lois Ruby Heather Christie
Heather Christie Lisa Caprelli
Lisa Caprelli Dmitry A Kondrashov
Dmitry A Kondrashov Jeremy Bernstein
Jeremy Bernstein Aubrey Graves
Aubrey Graves Barbara Dilorenzo
Barbara Dilorenzo B G Pachpatte
B G Pachpatte Athena Simone
Athena Simone Autumn Libal
Autumn Libal Dana Summer
Dana Summer Barbara Barbieri Mcgrath
Barbara Barbieri Mcgrath Traci Glover Walker
Traci Glover Walker
Light bulbAdvertise smarter! Our strategic ad space ensures maximum exposure. Reserve your spot today!

 Earl WilliamsIdeals, Varieties, and Algorithms: Your Essential Guide to the Foundations of...
Earl WilliamsIdeals, Varieties, and Algorithms: Your Essential Guide to the Foundations of... Angelo WardFollow ·7.3k
Angelo WardFollow ·7.3k Bret MitchellFollow ·6.1k
Bret MitchellFollow ·6.1k Colt SimmonsFollow ·7.8k
Colt SimmonsFollow ·7.8k Jan MitchellFollow ·4k
Jan MitchellFollow ·4k Ashton ReedFollow ·19.9k
Ashton ReedFollow ·19.9k Shane BlairFollow ·14.3k
Shane BlairFollow ·14.3k Ralph Waldo EmersonFollow ·11.7k
Ralph Waldo EmersonFollow ·11.7k Rob FosterFollow ·19k
Rob FosterFollow ·19k

 Marcus Bell
Marcus BellHigh Lonesome: A Literary Journey into the Heart of the...
<p>Hannah weaves a intricate...

 Gabriel Hayes
Gabriel HayesRediscover Gideon Green's Timeless Adventures in "Gideon...
Embark on an Extraordinary Journey with...

 Samuel Taylor Coleridge
Samuel Taylor ColeridgeEscape to a Literary Haven: Discover the Enchanting World...
Embark on an Extraordinary Literary...
5 out of 5
| Language | : | English |
| File size | : | 46727 KB |
| Text-to-Speech | : | Enabled |
| Enhanced typesetting | : | Enabled |
| Print length | : | 328 pages |
| Lending | : | Enabled |
| Screen Reader | : | Supported |
| Paperback | : | 68 pages |
| Item Weight | : | 4.8 ounces |
| Dimensions | : | 7 x 0.16 x 10 inches |