Mastering the Art of Writing Reaction Mechanisms in Organic Chemistry

Organic chemistry is a vast and intricate field that explores the structure, properties, and reactions of carbon-containing compounds. One of the most fundamental aspects of organic chemistry is the ability to understand and depict chemical reaction mechanisms. A reaction mechanism provides a step-by-step account of how a chemical reaction occurs, elucidating the sequence of events that lead to the formation of products.
4.8 out of 5
| Language | : | English |
| File size | : | 28504 KB |
| Print length | : | 488 pages |
| Screen Reader | : | Supported |
Writing reaction mechanisms is an essential skill for any aspiring organic chemist. It requires a deep understanding of organic chemistry principles, including molecular structure, bonding, and reactivity. The ability to accurately depict reaction mechanisms is crucial not only for academic pursuits but also for various practical applications, such as drug design, materials science, and environmental chemistry.
The Importance of Reaction Mechanisms
Reaction mechanisms play a pivotal role in organic chemistry for several reasons:
- Prediction of Reactivity: Understanding reaction mechanisms allows chemists to predict the reactivity of different functional groups and molecules. By analyzing the mechanism, chemists can identify the key steps that determine the reaction rate and selectivity.
- Design of Synthetic Strategies: Reaction mechanisms guide the design of synthetic strategies to achieve desired products. Chemists can devise efficient and selective synthetic pathways by manipulating the reaction conditions and choosing suitable reagents based on the mechanistic insights.
- Interpretation of Experimental Data: Reaction mechanisms help interpret experimental data and troubleshoot synthetic procedures. By comparing the observed results with the predicted mechanism, chemists can identify potential problems or optimize the reaction conditions.
- Understanding Biological Processes: Many biological processes involve organic reactions. Elucidating the reaction mechanisms underlying these processes is crucial for understanding their regulation and developing new therapeutic interventions.
Principles of Reaction Mechanism Writing
Writing reaction mechanisms involves several key principles:
- Identify the Functional Groups: The first step is to identify the functional groups involved in the reaction and determine their reactivity based on their electronic properties.
- Identify the Initiating Event: Most organic reactions require an initiating event, such as the formation of a reactive intermediate or the cleavage of a bond. Identifying the initiating event helps establish the starting point of the mechanism.
- Draw the Electron Flow: The heart of a reaction mechanism is the depiction of electron flow through the various intermediates. This involves showing the movement of electrons as bonds are formed and broken.
- Use Curved Arrows: Curved arrows are used to indicate the movement of electrons. The direction of the arrowhead points from the electron donor to the electron acceptor.
- Include All Intermediates and Transition States: A complete reaction mechanism should include all the intermediates and transition states involved in the process. Intermediates are species with distinct structures that exist transiently during the reaction, while transition states are high-energy species that represent the maximum energy point along the reaction path.
- Balance the Mechanism: The reaction mechanism should be balanced with respect to atoms and charges. All electrons and atoms must be accounted for throughout the mechanism.
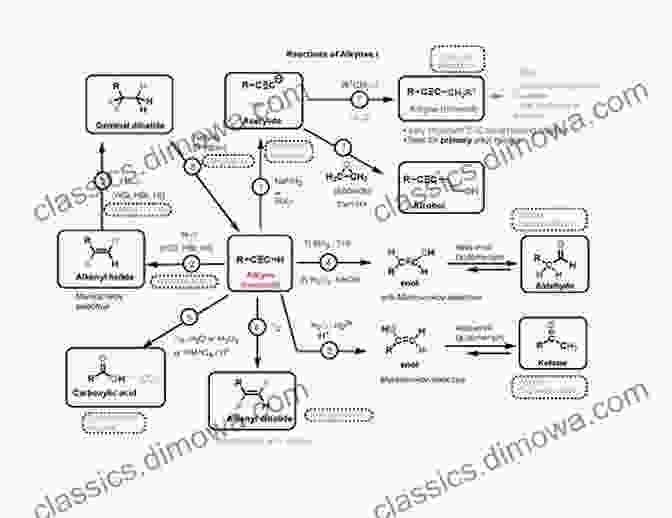
Common Reaction Mechanisms
Organic chemistry encompasses a vast array of reaction mechanisms. Some of the most common and widely studied mechanisms include:
- Nucleophilic Substitution: Nucleophilic substitution occurs when a nucleophile (an electron-rich species) attacks an electrophile (an electron-deficient species),leading to the replacement of a leaving group.
- Electrophilic Addition: Electrophilic addition involves the addition of an electrophile to a multiple bond, typically a double or triple bond.
- Elimination Reactions: Elimination reactions result in the removal of two substituents from adjacent carbon atoms, leading to the formation of a double or triple bond.
- Pericyclic Reactions: Pericyclic reactions involve concerted rearrangements of electrons within a cyclic system without the formation of intermediates.
- Radical Reactions: Radical reactions involve species with unpaired electrons, known as free radicals. These reactions often proceed via a chain mechanism with multiple radical intermediates.
Importance of Practice
Mastering the art of writing reaction mechanisms requires consistent practice. The more mechanisms you write, the better you will become at identifying the key steps and depicting the electron flow accurately. It is essential to start with simple mechanisms and gradually progress to more complex ones as your understanding deepens.
Working through practice problems and exercises can significantly improve your skills. Textbooks, online resources, and problem sets specifically designed for reaction mechanism writing can provide invaluable guidance. Collaborating with peers or seeking feedback from instructors can also help refine your understanding and identify areas for improvement.
Writing reaction mechanisms in organic chemistry is a fundamental skill that empowers chemists to understand and predict chemical reactivity, design synthetic strategies, interpret experimental data, and unravel the complexities of biological processes. By following the principles outlined in this article, practicing consistently, and seeking support when needed, you can develop your skills and become proficient in this essential aspect of organic chemistry.
Remember, the journey of mastering reaction mechanism writing is not just about memorizing specific mechanisms but about developing a deep conceptual understanding of organic chemistry principles. With dedication and practice, you can unlock the secrets of chemical reactions and unlock a world of possibilities in this fascinating field.
4.8 out of 5
| Language | : | English |
| File size | : | 28504 KB |
| Print length | : | 488 pages |
| Screen Reader | : | Supported |
Do you want to contribute by writing guest posts on this blog?
Please contact us and send us a resume of previous articles that you have written.
 Book
Book Novel
Novel Page
Page Chapter
Chapter Text
Text Story
Story Genre
Genre Reader
Reader Library
Library Paperback
Paperback E-book
E-book Magazine
Magazine Newspaper
Newspaper Paragraph
Paragraph Sentence
Sentence Bookmark
Bookmark Shelf
Shelf Glossary
Glossary Bibliography
Bibliography Foreword
Foreword Preface
Preface Synopsis
Synopsis Annotation
Annotation Footnote
Footnote Manuscript
Manuscript Scroll
Scroll Codex
Codex Tome
Tome Bestseller
Bestseller Classics
Classics Library card
Library card Narrative
Narrative Biography
Biography Autobiography
Autobiography Memoir
Memoir Reference
Reference Encyclopedia
Encyclopedia Barbara I Bond
Barbara I Bond Barry Ahern
Barry Ahern Sandy Mckay
Sandy Mckay Barry Flicker
Barry Flicker W Bolton
W Bolton Barb Geiger
Barb Geiger B R Paulson
B R Paulson Artyom Dereschuk
Artyom Dereschuk Aurelia D Andrea
Aurelia D Andrea Barbara J Gilbert
Barbara J Gilbert Kathy Macmillan
Kathy Macmillan Helen Crowley Cheek
Helen Crowley Cheek Michelle Young
Michelle Young Barbara Raue
Barbara Raue Marian Scadden
Marian Scadden Barry Cannon
Barry Cannon Clark Norton
Clark Norton Barney Norris
Barney Norris Dawn Schildhorn Esq
Dawn Schildhorn Esq Fintan Walsh
Fintan Walsh
Light bulbAdvertise smarter! Our strategic ad space ensures maximum exposure. Reserve your spot today!

 Fyodor DostoevskyThe Nile Tales: An Enchanting Photographic Journey into the Heart of Ancient...
Fyodor DostoevskyThe Nile Tales: An Enchanting Photographic Journey into the Heart of Ancient...
 Braeden HayesAggregation Functions Encyclopedia: A Gateway to the Realm of Mathematical...
Braeden HayesAggregation Functions Encyclopedia: A Gateway to the Realm of Mathematical...
 Stephen FosterUnlock Your Bar Exam Success with "February 2024 CA Bar Exam Essay Questions...
Stephen FosterUnlock Your Bar Exam Success with "February 2024 CA Bar Exam Essay Questions... Lucas ReedFollow ·11.3k
Lucas ReedFollow ·11.3k Paul ReedFollow ·17.9k
Paul ReedFollow ·17.9k Johnny TurnerFollow ·8k
Johnny TurnerFollow ·8k Mario BenedettiFollow ·11.4k
Mario BenedettiFollow ·11.4k Devin CoxFollow ·7k
Devin CoxFollow ·7k Adrien BlairFollow ·7.9k
Adrien BlairFollow ·7.9k Marc FosterFollow ·14k
Marc FosterFollow ·14k Alexander BlairFollow ·5.2k
Alexander BlairFollow ·5.2k

 Marcus Bell
Marcus BellHigh Lonesome: A Literary Journey into the Heart of the...
<p>Hannah weaves a intricate...

 Gabriel Hayes
Gabriel HayesRediscover Gideon Green's Timeless Adventures in "Gideon...
Embark on an Extraordinary Journey with...

 Samuel Taylor Coleridge
Samuel Taylor ColeridgeEscape to a Literary Haven: Discover the Enchanting World...
Embark on an Extraordinary Literary...
4.8 out of 5
| Language | : | English |
| File size | : | 28504 KB |
| Print length | : | 488 pages |
| Screen Reader | : | Supported |